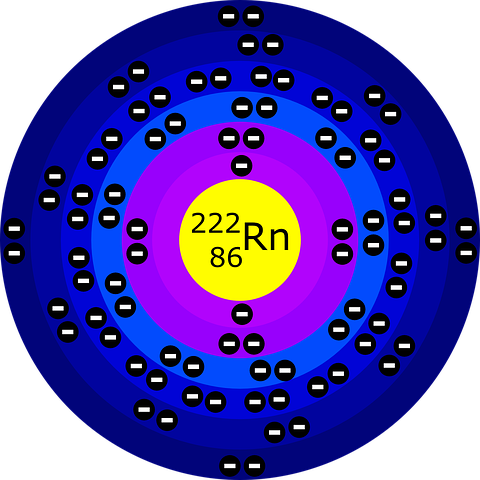
The history of the atom dates back to ancient Greece, where philosophers like Democritus and Leucippus proposed that all matter was made up of tiny, indivisible particles called atoms. However, this idea was purely speculative and lacked any empirical evidence.
It wasn't until the 18th and 19th centuries that scientists began to develop experimental methods for studying atoms. In 1803, English chemist John Dalton proposed a theory of atomic structure based on his experiments with gases. He suggested that all matter was composed of small, indivisible particles called atoms, and that these atoms had specific properties such as mass and chemical reactivity.
In the late 19th century, scientists began to discover subatomic particles such as electrons and protons. In 1897, J.J. Thomson used a cathode ray tube to discover the electron, a negatively charged subatomic particle that orbits the nucleus of an atom. In 1911, Ernest Rutherford conducted the famous gold foil experiment, which showed that atoms have a small, positively charged nucleus at their center, surrounded by negatively charged electrons.
Throughout the early 20th century, scientists continued to study the properties and behavior of atoms. In 1932, James Chadwick discovered the neutron, a neutral subatomic particle that also exists within the nucleus of an atom. The discovery of the neutron helped explain many properties of atoms, including their radioactive behavior.
Today, our understanding of the atom is based on the field of quantum mechanics, which describes the behavior of subatomic particles and their interactions with one another. Our understanding of the atom has also led to the development of many technologies and applications, including nuclear power, medical imaging, and materials science.
The concept of the atom, as the smallest building block of matter, has a long and fascinating history. Here is a brief overview:
The Greek philosopher Democritus (460-370 BCE) first proposed the idea of the atom, suggesting that all matter was made up of tiny, indivisible particles called atoms.
However, it wasn't until the late 1800s that the modern understanding of the atom began to take shape, with the work of scientists such as John Dalton, who proposed that atoms were the basic unit of chemical reactions, and J.J. Thomson, who discovered the electron and proposed the "plum pudding" model of the atom, with negatively charged electrons embedded in a positively charged sphere.
In 1911, Ernest Rutherford conducted the famous gold foil experiment, in which he fired alpha particles at a thin sheet of gold foil. He found that some of the particles were deflected, leading him to propose that atoms had a small, dense, positively charged nucleus at their center, surrounded by electrons in a cloud.
Niels Bohr further developed the atomic model in 1913, proposing that electrons traveled in specific, quantized energy levels around the nucleus. This model helped explain the spectral lines of various elements, which were caused by electrons moving between energy levels.
In the 1920s, quantum mechanics began to emerge as a new way of understanding the behavior of atoms and subatomic particles. Scientists such as Werner Heisenberg and Erwin Schrödinger proposed new models that took into account the wave-like behavior of particles at the atomic scale.
Over the years, scientists have continued to refine their understanding of the atom and its properties, leading to important discoveries in fields such as nuclear physics, chemistry, and materials science.
The significance of the atom lies in its fundamental role in understanding the physical and chemical properties of matter. By studying the behavior of atoms and their interactions with one another, scientists have been able to explain a wide range of phenomena, from the behavior of gases to the structure of crystals. In addition, the discovery of the nucleus and the development of nuclear physics has led to important advances in energy production and medical imaging, among other fields. Overall, the study of the atom has had a profound impact on our understanding of the natural world and our ability to manipulate it for our own purposes.